Lactic acid doesn't cause muscle pain. In fact, there's almost no lactic acid in your muscles!
The last time you were at the gym or helping a friend move, did you reach a point where your muscles felt like they were on fire? Before you blame lactic acid for the pain, you should consider that some of the “facts” you were taught in school were inaccurate or incomplete— lactic acid is one of the most common false teachings.
 |
The commonly accepted explanation for muscle pain begins factually with the burning (or “metabolism”) of sugar. At the start of your workout, sugar and oxygen are combined to generate energy for your muscles to use, but as your workout progresses, oxygen becomes a scarce commodity. Without oxygen, your cells cannot burn sugars 100 percent, so sugars are only burnt halfway in a process called anaerobic oxidation. And here’s where the science myth gets things wrong: the end result of anaerobic oxidation is not lactic acid but lactate.
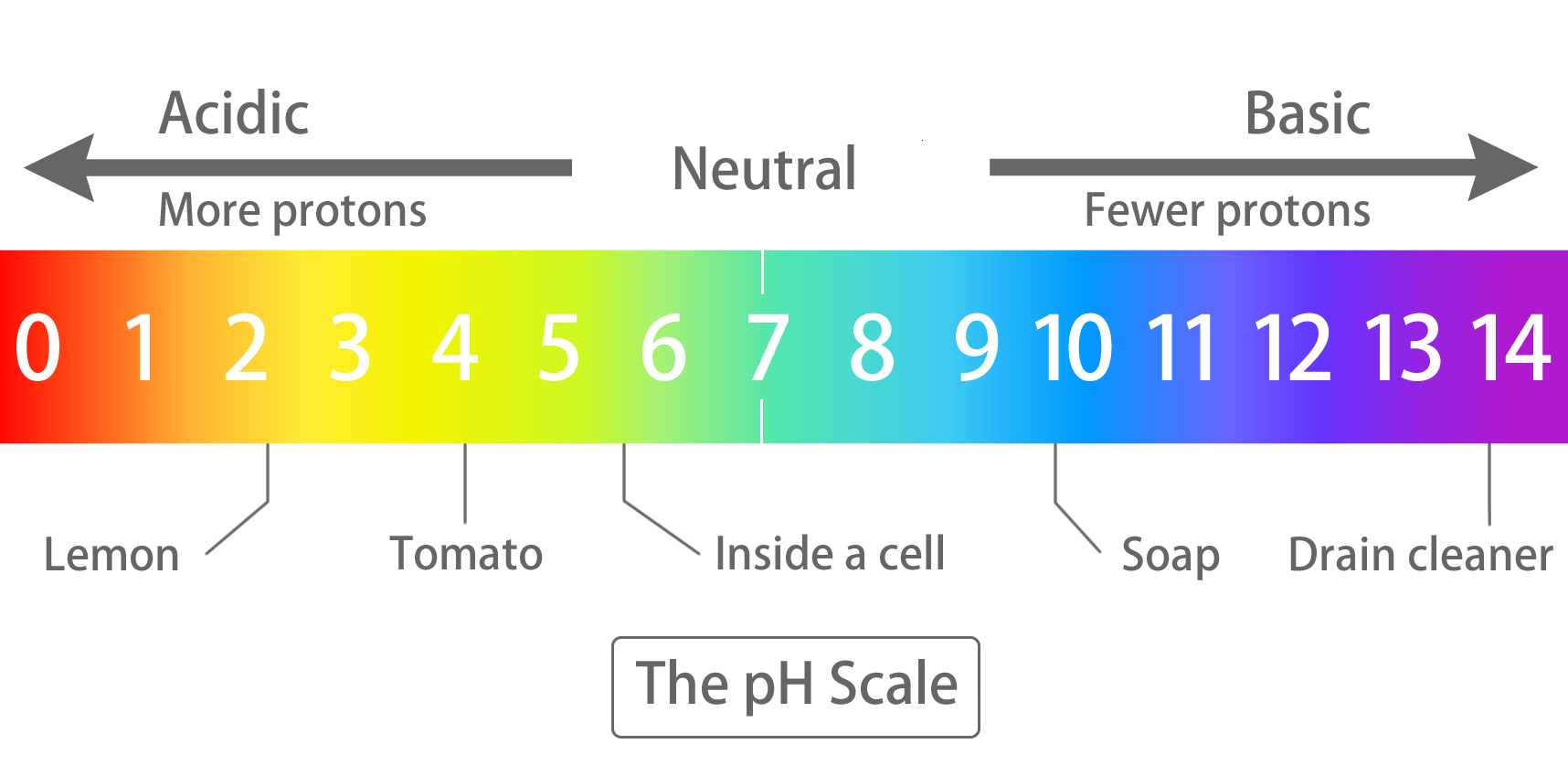
More accurately, the product is 99.03 percent lactate. Lactic acid and lactate are two sides of the same coin; which form dominates depends on the pH conditions of the cell. The lower the pH, the more lactic acid is converted to lactate, and vice versa. In order for lactic acid to outweigh lactate, the pH of the cell would have to be less than 3.86 (about as acidic as a tomato). In a normal cell, however, the pH is 5.99, so only 0.07 percent of the lactate is converted to lactic acid.
 |
That tiny value of 0.07 percent shrinks even further if you consider the reaction that creates lactate. To make one molecule of lactate, your cells require three molecules: pyruvate, NADH, and a proton.
Protons are the units we use to measure how acidic or basic a liquid is. Lots of protons = very acidic, few protons = neutral or even basic. Because the lactate-forming reaction uses up protons, it raises the pH of the cell, causing even more lactic acid to convert to lactate.
To top it all off, lactic acid is actually used as fuel in the muscle by transforming it back into glucose and burning it for energy again.
So what actually causes muscle pain, if not lactic acid? The verdict's still out on that, but the pain is likely caused by a combination of inflammation and tears in the connective tissue. In the meantime, let’s stop perpetuating the myth of lactic acid.








Facebook comments